Neurogenetics
The Department of Neurogenetics investigates the development of the mammalian nervous system and its age-dependent changes that are associated with disease. Using the tools of experimental mouse genetics and cell biology, our special focus is on the interaction of neurons and their axonal projections with neighboring glial cells, which are poorly understood. This provides the opportunity to discover novel cellular functions and mechanisms by which the cells of the nervous system interact and which are associated with a range of neuropsychiatric diseases.
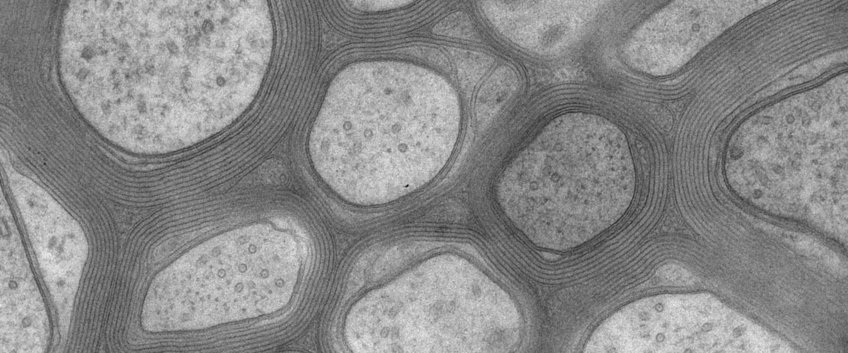
Two highly specialized glial subtypes, termed oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (PNS), serve the function of making myelin. By wrapping axons with a multi-layered sheath of myelin membrane, these glial cells insulate axons electrically and enable the fast and efficient propagation of electrical impulses. Myelin is a requirement for our normal motor, sensory, and cognitive functions. But how do glial cells and axons recognize and communicate with each other during nervous system development and later in the adult brain?
We have discovered the axonal signals in the PNS that regulate myelin growth by Schwann cells, a protein termed NRG1 type III. However, the identity and function of corresponding molecules driving the myelination by oligodendrocytes is still mysterious. And what genes have led to myelination during vertebrate nervous system evolution to begin with? How do oligodendrocytes detect the electrical activity and the caliber of the axons they ensheath in order to make the proper amount of myelin? How are these processes controlled in space and in time? Is myelination of the forebrain ‘suppressed’ during early postnatal development in order to allow the maturation of more complex neuronal networks, i.e. the consolidation of postnatal experience in the cortical circuitry?
Myelination is also a ‘risk’ for the axon itself, because it entails the physical shielding of the axonal metabolism from the extracellular milieu and from unrestricted access to nutrients. Indeed, we found that myelinating glial cells themselves become responsible for the long-term integrity of the axons they engulf. We further discovered that oligodendrocytes contribute to the energy metabolism of the myelinated axon. With the end of active myelination, they switch their own intermediate metabolism such that the imported glucose is only used for the generation of lactate, which is then provided to the axon for further metabolism and the generation of ATP by mitochondria. In myelinating glial cells, this necessitates an intricate architecture of the sheath, with a system of narrow cytosolic channels that connect the glial cell body with the axon underneath the myelin sheath. Is this architecture subject to age-dependent degenerative changes? Are the morphological changes that one can visualize by electron microscopy perhaps a previously overlooked risk factor for cognitive decline in the aging brain and for the development of neurodegenerative diseases?
Our research on the interaction between neurons and glial cells in the circuitry of the mammalian nervous system thus addresses fundamental new questions in neurobiology. It also helps to better understand neurological and psychiatric diseases and may lead to new therapeutic strategies by rational design.

