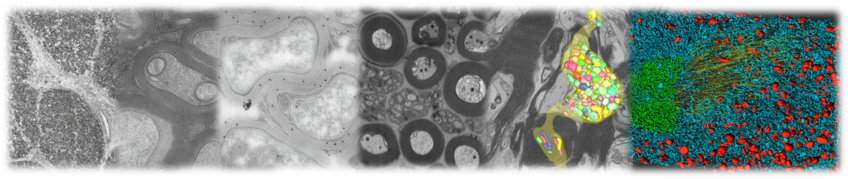
Facility for Electron Microscopy
City Campus
Our mission
The Facility for Electron Microscopy at the City Campus is part of the Department of Neurogenetics (Director: Klaus-Armin Nave). We work in the field of biological ultrastructural research in cooperation with scientists within the Max Planck Institute for Multidisciplinary Sciences and the Göttingen Science Campus in association with the Cluster of Excellence "Multiscale Bioimaging: from Molecular Machines to Networks of Excitable Cells" (MBExC).
What we offer
In our facility, we offer transmission electron microscopy (TEM) to analyse ultrastructure at subcellular resolution. In addition, we enable the generation of three-dimensional data sets (“volume electron microscopy”) using focused ion beam-scanning electron microscopy (FIB-SEM).
How we work
We work on a cooperative basis with scientists at the institute. We advise our partners on questions of sample fixation or carry this out ourselves. The subsequent sample preparation with embedding and preparation of the sections is performed entirely by our team. Interested doctoral students can also obtain training. The acquisition of transmission electron microscope images is carried out either by us or by the partners themselves. Data acquisition on the scanning electron microscope is performed exclusively by our team. We offer advice and assistance for the subsequent data analysis.
Press releases & research news
Exchange of information within the EM community
"Submicron" mailing list
Anyone working in the field of electron microscopy is often confronted with problems or questions that could be solved by talking to experienced colleagues. For this purpose, we launched an international e-mail list "submicron-list" to enable a quick exchange of information. This platform is also ideal for announcing conferences, workshops or job vacancies. Here you can subscribe to the list:
https://listserv.gwdg.de/mailman/listinfo/submicron-l
DGE (German Society of Electron Microscopy) and the PANOS working group
The PANOS (Preparation and Imaging of Native Organic Systems) working group of the German Society for Electron Microscopy (DGE) is organised by members of the DGE who work in the field of biomedical electron microscopy. We organise regular annual spring meetings to promote exchange and discuss interesting topics. This meeting is open to non-members of the DGE.
https://www.dge-homepage.de/
Information is exchanged via a mailing list: https://listserv.gwdg.de/mailman/listinfo/panos-list
