Spontaneous Closing of the Exportin Cse1p
Collaborations: Peter Hinterdorfer (Johannes-Kepler-Universität Linz, Austria) and Ziv Reich (Weizmann Institute of Science, Rehovot, Israel)
Financial Support: Human Frontier Science Program (HFSP)
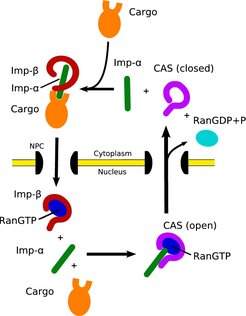
The transport of proteins and RNA through the nuclear pore complex is a key process in eukaryotic cells. It is mainly mediated by the superfamily of β-karyopherins, to which the exportin CAS (whose yeast homolog is Cse1p) belongs. During the transport cycle the exportin CAS undergoes a substantial conformational change from the open nuclear cargo and Ran-bound state to the closed cytoplasmic state. We studied these conformational changes applying molecular dynamics simulations. Our simulations revealed that the transition from the open state to the closed state occurs spontaneously on the very short time-scale of only a few nanoseconds, supporting the theory of a 'spring-loaded molecule'. Furthermore, from our simulations we derived the complete pathway of state transition. Transition was initiated by enhanced dynamics of the N-terminal HEAT repeats 1-3, followed by electrostatic attraction of the interface regions. The final shape was obtained by a domino-like relaxation of HEAT-repeats between the interfaces. This pathway is in contrast to previously assumed independent motion of two linked arches.
In the classical import cycle (Fig. 1) proteins carrying a nuclear localisation signal (NLS) bind to the soluble nuclear transport receptor importin-β via the adaptor protein importin-α. The resulting import complexes are translocated through the nuclear pore complex (NPC). Once the complex arrives in the nucleus, RanGTP binds to importin-β, thus triggering the release of its cargo.
In the nucleus, importin-α is sequestered by RanGTP and the transport receptor CAS, a close relative of importin-β. This heterotrimeric complex is then transported to the cytoplasm, where the Ran-bound GTP is hydrolysed to RanGDP, leading to the dissociation of CAS and importin-α, which becomes available for a new NLS-protein import round.

The most important class of NTRs are β-karyopherins to which importin-β and CAS belong. The structures of importin-β and CAS are very similar, both are formed by stacked HEAT repeats. A HEAT repeat is a hairpin structure, consisting of two α-helices and a central loop (pink shaded area in Fig. 2, top). This modular construction scheme leads to an extended, superhelical shape lacking a sizable globular hydrophobic core, which would usually confer rigidity to soluble proteins or protein domains.
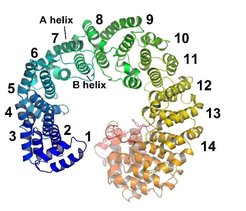
Therefore, and also due to the fact that the crystal structures of karyopherins show some variability in their shape depending on their binding partners, a high level of inherent flexibility is attributed to these proteins. This high flexibility of NTRs may help to recognize and bind cargo as well as facilitate transitions in the transport cycle.
High flexibility of CAS: We have tested the assumption of high flexibility on the exportin CAS (whose yeast homolog is Cse1p). To our surprise, we found that the transition from the open cargo and Ran-bound nuclear state to the closed cytoplasmic state occurs spontaneously on a very short time-scale of only a few nanoseconds. This fast and spontaneous transition shows that the initial open state is highly strained, supporting the theory of a "spring-loaded molecule", formed by an extreme case of protein-protein induced fit.
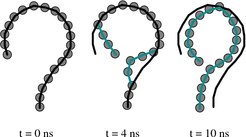
Using the open nuclear structure as the only input, we were able to predict the ring-shaped closed cytoplasmic state with a root mean squared deviation (RMSD) of only 3 Å from experimental data. The initiation of the process was seen to be brought about by enhanced dynamics of the N-terminal HEAT repeats 1-3, while completion of the closure was due to the electrostatic compatibility of the interface regions. We tested this initial pathway also by simulating mutants, and found that proteins with an altered electrostatic pattern failed to close. The final shape was obtained by a domino-like relaxation of HEAT repeats situated between the interfaces.
This transition pathway shows that the conformational variability of CAS is not based upon the independent motion of two centrally linked 'arches', as previously assumed, but rather on the enhanced flexibility and electrostatic properties of the terminal sections.



