PAINTing Drosophila muscles with nanobodies positions protein giants
Researchers succeeded to visualize Drosophila sarcomeres at super-resolution with the help of nanobodies and single molecule blinks.
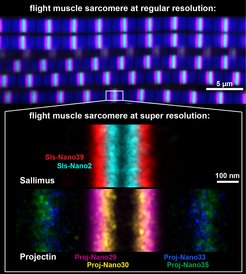
The image below is a collage of flight muscle sarcomeres imaged with nanobodies and DNA-PAINT at super-resolution. The ends of a short version of the titin homolog Sallimus are labeled in cyan (Sls start) and red (Sls end, whereas Projectin is labeled in magenta and yellow (Proj start), and in blue and green (Proj end). Each color was generated by a specific nanobody against a small part of the large proteins. Importantly the overlay of the positions of both proteins. The scale bar is 100 nm.
From humans to insects, muscles power body motions or pump body fluids. The force-producing unit in all muscles is called the sarcomere. All sarcomeres contain three key components: first, long parallel actin tracks; second, myosin motors that pull these actin tracks together to contract the sarcomere; and third, long titin spring proteins that mechanically connect actins with myosin and thus define the length of the mammalian sarcomere, in analogy to a ruler. An international collaboration led by Frank Schnorrer in Marseille, Dirk Görlich in Göttingen and Ralf Jungmann in Munich wanted to investigate if titin also rules the length of insect sarcomeres.
To achieve this, scientists have combined expertise to generate tools that specifically label the ends of the two different titin proteins present in the fruit fly, Drosophila melanogaster: with miniature versions of antibodies, called nanobodies, scientists were able to locate the ends of both fly titin homologs, called Sallimus and Projectin, with a precision of 5 nm. This high-resolution revealed the presence of short versions of the titin homologs in flight muscle sarcomeres showing an interesting staggered arrangement (see image). In contrast, the long sarcomeres in Drosophila larvae contain long versions of the Sallimus protein. The scientists concluded that, as in human muscles, the length and domain organisation of the insect titin versions likely determine the dimensions of insect sarcomeres in a muscle-type specific manner.
The overall architecture of sarcomeres is conserved from flies to humans, but not all its components are identical. Thus, it has been an open question how the dimensions of insect sarcomeres are determined by its components. An international collaboration funded by the European Research Council (ERC) now set out to study the exact architecture of insect sarcomeres. To visualise and measure sarcomeric components, Dirk Görlich and his team at the Max Planck Institute (MPI) for Multidisciplinary Sciences in Göttingen generated so-called nanobodies (miniaturized antibodies) that bind with high specificity to chosen regions in sarcomeric proteins. Attached labels let those nanobodies shine bright and colourful or blink under a fluorescence microscope.
Sarcomer components made visible
Vincent Loreau and Eunice Chan at the Developmental Biology Institute in Marseille tested these nanobodies in Drosophila muscles to verify their specific binding to the sarcomeric protein and located the position of the sarcomeric proteins in different muscle types of the fly. By using nanobodies that specifically recognise the beginning or the end of the Drosophila titin homolog Sallimus (Sls), the researchers showed that very long Sls versions (>2 micrometre) are present in larval Drosophila muscles that have very long sarcomeres, whereas as adult flight muscles that contain short sarcomeres only possess short Sls versions.
To measure the positions of the sarcomeric proteins more precisely, Florian Schueder in the group of Ralf Jungmann at the MPI of Biochemistry Munich, together with Pierre Mangeol in Marseille and Dirk Görlich in Göttingen modified the nanobodies such that they were suitable for a super-resolution microscope technique called DNA-PAINT microscopy. This technique allowed them to locate individual protein modules in sarcomeres with a precision of five nanometers.
Such, the exact locations of single modules of the two titin proteins could be measured precisely, which revealed that the end of the short Sallimus overlaps with the beginning of Projectin in the flight muscle sarcomeres (see image). This is interesting because it suggests that the two Drosophila titin homologs not only share the function that is encoded in the single large mammalian titin but their orientation suggests that both are also mechanically connected as is the large mammalian protein.
A unique advantage of nanobodies is that they can be tagged with fluorescent proteins and expressed in the living muscles of flies. Using this trick, Loreau and colleagues were able to report the dynamics of the fly titin Sallimus in larval muscles: They found that this protein is remarkably stable once incorporated into the sarcomere and is not replaced over the course of 30 minutes. Together, the combination of the here generated new tools will open new perspectives in sarcomere research to study the dynamics of sarcomere assembly and of sarcomere maintenance with unprecedented resolution in the Drosophila model.








