Effect of Sodium Chloride on Lipid Bilayers
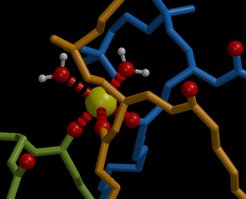
Increasing sodium chloride concentration was found to decrease the self-diffusion of POPC lipids within the bilayer. Self-diffusion coefficients calculated from the 100 nanosecond simulations agree with those measured on a millisecond time scale using fluorescence correlation spectroscopy, suggesting that most of the relaxation processes relevant for lipid diffusion are faster than the simulation time scale. As the dominant effect, the molecular dynamics simulations revealed a tight binding of sodium ions to the carbonyl oxygens of in average three lipids leading to larger complexes with reduced mobility. Additionally, the bilayer thickens by approx. 2 Å which increases the order parameter of the fatty acyl chains. Sodium binding alters the electrostatic potential which is largely compensated by a changed polarization of the aqueous medium and a lipid dipole reorientation.
Dynamics simulation of lipid bilayers
The movie below shows a bilayer system with 512 POPC lipids. A pre-equilibrated POPC bilayer (kindly provided by Peter Tieleman) was used as a start structure. All simulations were performed in a periodic box filled with more than 20.000 water molecules, corresponding to a hydration level of approx. 40 waters/lipid, yielding a total system size of more than 80.000 atoms. All MD simulations were carried out using the GROMACS simulation suite. Electrostatic interactions were calculated with the Particle-Mesh Ewald Method (PME). The temperature was coupled to an external temperature bath at 300 K.
Electrostatic potential across a POPC bilayer
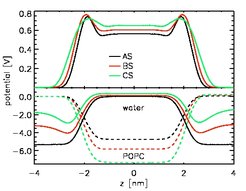
The total electrostatic potential is only slightly affected by the binding of sodium ions to the lipid headgroups (upper panel). However, the individual contributions (lower panel) differ significantly: The potential drop due to the lipid headgroups is increased, whereas the compensation due to the orientation of water dipoles is decreased. The potential contribution caused by the water dipoles exhibits an additional minimum when NaCl is present. Overall, the region of anisotropic dipole orientation is increased. Therefore, we expect an increased range of hydration phenomena is the presence of salt ions.


