Transient Kinetics of the Intrinsically Disordered Protein α-Synuclein: from Picosecond to Millisecond Time Scales
Collaborators: Elisha Haas, The Goodman Faculty of Life Sciences, Bar-Ilan University, Israel
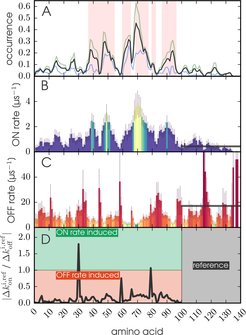
Figure 1: A) Relative occurrence of the secondary structure content for T-REX trajectories (blue), long WT trajectories (green) and weighted with a 40/60 ratio (black). Highlighted background (red) shows aS fibril β-sheet locations (PDB: 2N0A). B) Free monomeric aS β-sheet formation k and C) β-sheet dissociation rates k with error bars at 2 σ. Colors equal rates. D) Ratio between kon and koff relative to the unstructured C-terminus (reference). Ratios smaller than 1 indicate structure formation due to changes in the koff rates, ratios larger than 1 indicate formation due to changes in kon.
© R. Klement, MPI-NAT
Funding: Deutsche Forschungsgemeinschaft
Aggregation of α-Synuclein (aS) in the human brain is linked to the onset of Parkinson’s disease. Time scales of transient secondary structure formations and tertiary structure reorganization of free aS monomers prior to aggregation are still unknown. In particular, the interaction of the charged termini in the disordered state has been suggested to prevent permanent secondary structure formation in the fibril core region (residues 61-95), which was suggested to already transiently form β-sheets with unknown rates.

Figure 2: A) Residue contact map with N-C terminus contact region (black). B) Mono-exponential amino acid correlation times for short and long range correlations between the transient structure forming regions (top bar) in aS.
© R. Klement, MPI-NAT
In the absence of a stable fold, transient secondary structure kinetics define the native state of the prototypical and pharmacologically relevant intrinsically disordered protein (IDP) α-Synuclein.

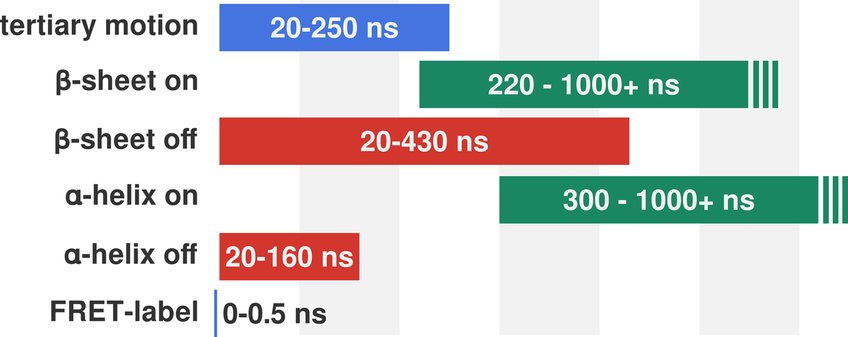
Figure 3: Overview of the observed time scales for tertiary motion and FRET labels (blue) and the secondary structure formation (green) and dissociation (red) time scales from 20 x 3 μs AMBER03ws/TIP4P2005s simulation trajectories.
© R. Klement, MPI-NAT
The intrinsic time scales are not only most relevant for the association of aS monomers into low molecular weight oligomers and further for the aggregation kinetics of fibril formation, but also for the interpretation of single molecule FRET experiments. Specifically, the relation between structure reorganization (ns-μs), dye dynamics (ps-ns), dye excitation decay (ns) and inter photon correlation times (ms) is largely unknown, but dictates the quantitative interpretation of FRET experiments.
For our studies, we generated structural ensembles of aS with four different force fields and water model combinations (amber99sb/tip3p, charmm22*/tip3p, charmm22*/tip4pD, amber03ws/tip4p2005s) with unrestrained temperature replica exchange Molecular Dynamics simulations and compared the ensemble parameters to data from NMR, time-resolved FRET and SAXS experiments. For the kinetic analysis, we used 20x3µs trajectories of wild type aS generated with the amber03ws force field that yielded best agreement with experimental data.
We investigated the kinetics preventing ordering and possibly pathogenic β-sheet aggregation. Interestingly, transient β-sheets form frequently at sub μs time scales precisely at the positions observed in aS amyloid fibrils. The formation kinetics competes with rapid secondary structure dissociation rates, thus explaining the low secondary structure content. The fast secondary structure dissociation times are very similar to the dynamics of tertiary structure rearrangements. These findings suggest that the fast dissociation kinetics slows down conformational selection processes for aS aggregation, which may be a common mechanism controlling the aggregation kinetics of IDPs.
Publication
Transient secondary and tertiary structure formation kinetics in the intrinsically disordered state of α-Synuclein from atomistic simulations. ChemPhysChem 19 (19), pp. 2507 - 2511 (2018)


