Tension Induced Titin Kinase Activation
Is titin kinase the force sensor of the muscle cell?

Activation of the titin kinase, the catalytic domain of the muscle protein titin, requires major conformational rearrangements resulting in the exposure of its phosphorylation site. It can be assumed but has not yet been shown that the requisite structural change is caused by stretched titin passing down the tension to the titin kinase. Force probe molecular dynamics simulations can give a detailed description of the activation mechanism and, hence, can test the hypothesis that titin kinase is the force sensor for the muscle cell.

Titin, a filament of the muscle cell, is a giant protein of approx. 3000 kDa, spans half the sarcomere, and is the longest covalently linked protein known [1]. Titin is composed of approx. 300 repeating domains, which mainly are similar to immunoglobulin and fibronectin, and one enzymatic domain: the titin kinase (Fig. 1).

The titin filament unfolds upon muscle cell stretching and refolds upon relaxation, thereby realigning the sarcomere and giving the muscle cell its elasticity [1] (Fig. 2). A crystal structure of the titin-kinase domain is solved and shown in Fig. 3 [2]. Activation of the titin kinase requires release of the auto-inhibitory tail from the active site.
Located near the C-terminus the titin kinase is under the influence of tension of unfolded titin. An obvious assumption is that the active force of the filamentous titin molecule triggers this major conformational change in the titin kinase. In other words, in response to a force of certain magnitude, partly unfolded titin domains could pass down the mechanical stress to the titin kinase. The subsequent structural rearrangement could relieve the autoinhibition.
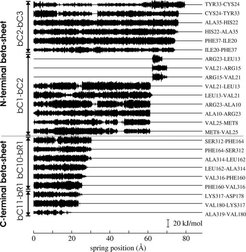
Our simulations aimed at understanding the molecular mechanism and energetics of the titin kinase activation induced by the pulling force of a titin molecule in a stretched muscle cell. Force probe molecular dynamics were used to complement the experimental results of atomic force microscopy on titin kinase ([3], for AFM of other titin domains see [4]). Our results suggest the rupture of two terminal -sheets as the primary unfolding steps. Their different force resistance, crucial for the activation, was attributed to their contrastive topology. This finding confirmed the general concept that the mechanical stability of proteins with mechanical function is achieved and controlled by specific structural and topological properties.
Our results support the hypothesis of titin kinase as a force sensor, fulfilling its function via an unfolding motion thoroughly designed to convert mechanical load into a biochemical signal. Indeed, more recent studies suggest a direct link between the mechanical load in a muscle cell and changes in the distribution between titin and the nucleus of transcription factors downstream in the titin kinase signalling pathway [5], corroborating the concept of titin sensoring force even further.
Movies
Publication
References
SCIENCE 276 (5315), 1109-1112 (1997)





