Photoswitching Mechanism of the Fluorescent Protein asFP595
Collaborations: Stefan Hell (MPI-NAT) and Markus Wahl (FU Berlin), Matthias Ullmann (University of Bayreuth), and Mike Robb (Imperial College London)
Supported by: Boehringer Ingelheim Fonds

The discovery of green fluorescent protein (GFP) in the early 1960s ultimately heralded a new era in cell biology. GFP can be tagged to subcellular structures, and the readout of its fluorescence emission with an optical microscope enables to monitor cellular processes in living organisms. Further, a photo-switchable fluorescent protein has been characterized, which opens new routes to subwavelength far field optical microscopy and biotechnology. The solution of its structure, both in the fluorescent 'on' and the 'off' state paved the way to study the photoswicthing mechanism at the atomic level by combined QM/MM simulations. Collaborating with other groups, we were able to identify a 'hula-twist' mechanism and triggered proton transfers between dye and adjacent residues as the mechanism underlying photo-switching. Understanding this mechanism allows to rationalize results from site directed mutagenesis as well as to suggest new mutants.
Optical microscopy based on GFP fluorescence is a powerful technique but its resolution is usually limited due to diffraction and photobleaching. However, resolution can be remarkably improved and susceptibility to photobleaching dimished with the availability of fluorescent proteins that can be selectively switched on and off.
The GFP variant asFP595 has been isolated from the sea anemone Anemonia sulcata (Figure 1), and its structure and spectroscopic properties have been determined. The asFP595 protein is an 'optical highlighter', i.e., its fluorescence emission can be optionally switched 'on' and 'off' in response to irradiation of a particular wavelength. These favourable photochromic properties not only enable one to circumvent photobleaching but also allow direct tracking of the movement of objects in living cells, and open the way for a systematic improvement of the spatial resolution of optical microscopes. In a proof-of-principle experiment, the Hell group has demonstrated that, using asFP595, spatial resolution can be dramatically improved to 50 nm (0.00005 mm) in the focal plane, which is far below the Abbe resolution of around 500 nm.
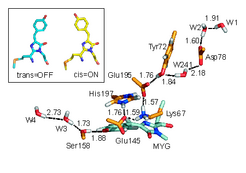
Classical molecular dynamics simulations revealed that asFP595 photoswitching involves a trans-cis isomerisation of the chromophore molecule according to a space-saving 'hula-twist' mechanism (Figure 2, inset). Additional mixed quantum/classical (QM/MM) calculations showed that the isomerisation triggers proton transfer events between the chromophore and adjacent amino acids.
This detailed understanding of the molecular mechanism underlying asFP595 photoswitching provides the first comprehensive picture of molecular photoswitching of proteins and enabled us to rationalize results from site directed mutation studies as well as to suggest new mutants with improved properties.

