Mechanical Energy Transduction in the F0F1-ATPase
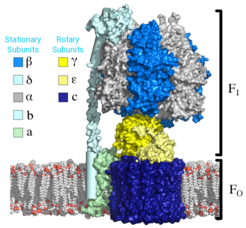
The F0F1 ATP Synthase is a complex nanomotor that synthesizes nearly 90% of the ATP made during cellular respiration. It consists of two coupled rotary motors: an integral membrane complex driven by proton flow across lipid bilayers (F0) and an enzymatic complex that converts ADP and inorganic phosphate to ATP (F1). The rotational portion of these motors acts as a crankshaft, inducing conformational changes that lead to ATP synthesis in the F1 motor’s three functional catalytic sites. The F1 motor can perform ATP synthesis in the absence of F0, and it can also work in reverse, hydrolyzing ATP to pump protons against an established gradient.
Over the last 30 years many important aspects of this motor’s function have been elucidated by careful biochemical work and further understood by clever biophysical experiments. However, there is still not a complete, quantitative description of the whole thermodynamic cycleone that fully describes the interactions between all three separate catalytic sites and accounts for the need to exchange ATP (found abundantly) for the relatively sparse ADP.
Our past work has helped better understand energy transfer, stability of the γ-subunit, and γ’s role in the synthesis mechanism. We are continuing to elucidate the conformational changes associated with the binding of ADP and phosphate and the release of ATP. We study both the stability of the isolated γ-subunit against torsion and its capacity to store energy as well as the energy transfer in the full ATPase.
The goal of the present project is to develop a model of the F1-ATPase function based on molecular dynamics (MD) simulations and computational modeling techniques. This model will be fundamentally different form earlier work in that all configurations will be considered, allowing for the possibility of multiple paths rather than imposing one. We hope to identify the precise interactions that define the power stroke and the transitions between functional states, determining how this nanomotor converts energy from chemical to mechanical and back to chemical form. Multiple MD simulations using the GROMACS package are being used to understand the interaction between γ and the catalytically-active β subunits. Additionally, we are developing in-house tools to aid in model construction.
Publications
References
Biochimica et Biophysica Acta - Bioenergetics 1757 (5-6), 286-296 (2006)

