Experimental drug interferes with different mechanisms associated to Alzheimer’s disease
The chemical compound anle138b eases cognitive deficits and normalizes gene expression in a mouse model of Alzheimer’s disease. Furthermore, the drug seems to close harmful openings in the membrane of nerve cells. An international collaboration reports these findings in the journal EMBO Molecular Medicine. The scientists suggest that anle138b should be validated in clinical trials for its potential to treat Alzheimer’s and possibly other neurodegenerative diseases. The study involved institutions from Europe and the US including the German Center for Neurodegenerative Diseases (DZNE), the University Medical Center Göttingen (UMG), the Max Planck Institute for Biophysical Chemistry (MPI-BPC), the Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB), Göttingen, the Technische Universität Braunschweig, and the University of California San Diego (UC San Diego).
Alzheimer’s is a devastating neurodegenerative condition ultimately leading to dementia. An effective treatment does not yet exist. The disease is associated with the aberrant aggregation of small proteins called Aß-peptides that accumulate in the brain and appear to harm neurons. However, the molecular events that lead to neurodegeneration are not entirely resolved.
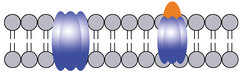
“According to one hypothesis, aggregates of Aß-peptides could be implicated in the formation of membrane channels that open a gateway to ions. Their uncontrolled exchange between a cell’s interior and exterior may alter intracellular ion levels. This could potentially trigger neuronal dysfunctions and may lead to cell death”, says Prof. André Fischer, a senior researcher at the DZNE and the UMG.
Pore formation in the spotlight
To test this hypothesis, both artificial and neuronal membranes were investigated in the current study. “Although a definitive proof is still missing, our work supports the assumption that pore formation due to Aß-peptides may indeed be involved in Alzheimer’s,” states Ratnesh Lal, a professor of bioengineering at UC San Diego.
Furthermore, the data suggest that although anle138b does not prevent pore formation it induces conformational changes, thereby modifying pore conductivity. This greatly reduces or, in most cases, eliminates ionic flux.
Tests in an animal model
Anle138b was synthesized at the MPI-BPC in the department of Prof. Christian Griesinger in collaboration with Armin Giese from the Ludwig-Maximilians-Universität Munich (LMU). In previous studies, the compound has been shown to prevent misfolding of certain proteins that play a role in neurodegenerative diseases. “Anle138b attaches to protein aggregates, thereby modifying their properties. The drug is able to reach the brain when taken orally. Therefore, it is easy to administer,” Griesinger explains.
In the current study, anle138b was given to mice affected by abnormal brain function, impaired memory, and Aß-peptides accumulating in their brains. Similar conditions manifest in Alzheimer’s patients. Treatment with anle138b normalized the rodents’ brain activity and improved their learning ability, irrespective of whether the intervention started before or after the onset of Aß deposition. “It came as a big surprise that this positive effect also took place when the disease was well advanced,” says Prof. Martin Korte of the Technische Universität Braunschweig.
Disease-modifying properties
Moreover, the researchers studied the drug’s action on gene expression. For this, they examined the expression levels of neurons of the hippocampus, a brain area involved in memory function. They found that numerous genes were deregulated in the diseased mice. However, anle138b treatment to a large extent reinstated gene expression and thus a healthy balance of proteins. This suggests that the substance not only mitigates symptoms but also affects the disease process and acts disease-modifying.
Effect on Tau proteins
These results are in agreement with earlier findings revealing that anle138b acts on a protein called Tau, a second protein involved in Alzheimer’s. These experiments showed that anle138b eased cognitive deficits in mice affected by Tau aggregation and inhibited Tau proteins from sticking together.
“Care has to be taken when interpreting all these results, because none of the current animal models fully recapitulates the symptoms seen in Alzheimer’s patients,” emphasizes Prof. Gregor Eichele, Director at the MPI-BPC.
“However, anle138b is certainly quite unique, as it interferes with both of the two major hallmarks of Alzheimer’s disease, namely Aß-peptides and Tau proteins. I would thus consider it a potential candidate for clinical trials on Alzheimer’s and perhaps other neurodegenerative conditions,” DZNE researcher Fischer adds.
Preparatory work is already in progress: Anle138b is to be developed for market rollout by MODAG, a joint spin-off of the LMU and the MPI-BPC. The researchers hope that anle138b might one day help to stop pathological conditions such as Parkinson’s, Alzheimer’s, and Creutzfeldt Jakob disease.
Joint press release of the DZNE, MPI-BPC, UMG, Technische Universität Braunschweig, and CNMPB

![[Fe]-hydrogenase catalysis visualized using para-hydrogen-enhanced nuclear magnetic resonance spectroscopy](/4868136/teaser-1734084319.jpg?t=eyJ3aWR0aCI6MzYwLCJoZWlnaHQiOjI0MCwiZml0IjoiY3JvcCIsImZpbGVfZXh0ZW5zaW9uIjoianBnIiwib2JqX2lkIjo0ODY4MTM2fQ%3D%3D--0262c0fa020d6ea4060dd5aacac2a1dcdee31120)









