Auditory Neuroscience & Synaptic Nanophysiology

The Auditory Neuroscience & Synaptic Nanophysiology group aims to decipher the mechanisms of synaptic transmission at the hair cell ribbon synapse and the giant calyceal synapses of the central auditory pathway, foremost the endbulb of Held synapse in the cochlear nucleus. We are interested in the mechanisms that enable the impressive temporal precision and long-lasting reliability of synaptic transmission in the early auditory pathway. We approach these questions by a systematic analysis of the molecular nanoanatomy and nanophysiology of hair cell ribbon synapses and endbulb of Held synapses in normal and molecularly manipulated mice.
We perform pre- and/or postsynaptic patch-clamp measurements to stimulate and record synaptic transmission at high resolution and to manipulate the synapse. Whole-cell and cell-attached measurements of membrane capacitance are used to report exocytic and endocytic membrane turnover in the presynapse. The apparent and intrinsic Ca2+ dependence of exocytosis is studied by manipulating the Ca2+ current and uncaging of caged Ca2+, respectively. Confocal and STED microscopy are applied for studies of molecular anatomy and physiology of these synapses.
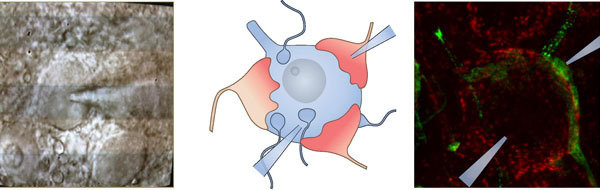
Molecular Nanoanatomy and Nanophysiology of inner hair cell ribbon synapses
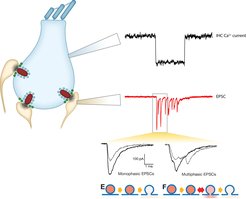
In our group, we aim to understand the molecular machinery of vesicle release at IHCs. Post-synaptic recordings enable us to monitor release of individual vesicles at single active zone (AZ) with high resolution. Simultaneous recording of IHC opens access to control conditions (e.g. membrane potential) of IHCs and to manipulate intracellular contents such as Ca2+, buffer proteins and synaptic proteins. The excitatory postsynaptic current (EPSC) can be classified into two categories according to its waveform: monophasic and multiphasic EPSCs. The different waveforms may represent different modes of release -- full collapse of vesicles and flickering of fusion pore. By careful analysis of EPSCs under different experimental conditions, we will resolve the molecular nanophysiology of vesicular pool dynamics, Ca2+ influx-excoytosis coupling and the release mechanisms at the IHC synapse. We also study the molecular nanoanatomy of these synapses.
The focus of functional imaging is on Ca2+ signaling and vesicle turnover at individual active zones. Imaging also builds on collaboration with the Dept. of Nanobiophotonics of Stefan Hell. We use the acquired morphological and functional data as input into our biophysical modeling that we perform in collaboration with Fred Wolf, Theoretical Neurophysics Group, MPI for Dynamics and Self-Organization and which in return helps us to further test our hypotheses and design new experiments. Funding comes from the Max-Planck-Society, the University Medical Center Göttingen and the DFG through the Collaborative Research Center 889 and the Research Center for Nanoscale Microscopy and Molecular Physiology of the Brain.

