A door opener for cell suicide
Researchers show how the protein Bax makes the cell’s decision to die irrevocable
When body cells kill themselves, this is done for a good reason. Damaged or pathologically altered cells can pose a threat to the organism. Through their death, the organism protects itself from neurodegenerative diseases, autoimmune diseases, and cancer. Also, an embryo can only develop healthily when cells die after they have completed their task. This cellular suicide program, once trigged, follows a strict course. The mitochondria, which supply the cell with energy, play a central role here. Should a cell start this apoptotic program, holes are induced in the mitochondria’s envelope. Thereby, proteins from the interior are released into the surrounding cytoplasm. As a result, a chain reaction is started, inevitably killing the cell.

Although we know many factors which are involved in making the mitochondrial membrane permeable, how this is done exactly has been unclear so far. A team of scientists led by Stefan Jakobs at the Max Planck Institute (MPI) for Biophysical Chemistry and the University Medical Center in Göttingen has now discovered that upon apoptosis the protein Bax is organized into annular rings on the mitochondria which helps to open up the membrane.
As long as the cell is healthy, the Bax proteins are located mostly in the cytoplasm. But when apoptosis begins, they change their molecular shape and concentrate in large numbers on the mitochondrial membrane. "Previous microscope images showed that Bax proteins form large aggregations which are anchored in the membrane," Stefan Jakobs explains. "But so far unanswered was the question: What is the function of these aggregations?" There was only a guess: Bax could in a sense act as "channel digger" in the mitochondrial membrane and create holes through which the apoptotic proteins could get into the cytoplasm. "However, so far, no one had found according arrangements of Bax in cells," says Jakobs.
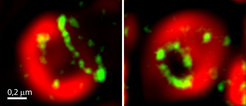
Using ultra-high resolution STED microscopy, Jakobs’ team has now managed to examine the distributions of Bax proteins with unprecedented detail. "In our high-resolution images it can be clearly seen that the Bax gatherings in reality often are rings of different sizes," says Daniel Jans, scientist in the Jakobs team. More elaborate experiments revealed another important detail: "Normally, the outer mitochondrial membrane is tightly packed with numerous proteins. But inside the Bax rings, these proteins are missing. This suggests that in the interior of the rings the membrane is displaced, implying a hole – like channels."
The scientists thus show for the first time that Bax proteins could form pores in the membrane of the mitochondria. The new findings provide an important contribution to better understand the mechanistic details of apoptosis. (Jaydev Jethwa/fk)













