Scientists unveil secrets of important natural antibiotic
Breaking out in sweat not only regulates our body temperature. Sweat also spreads highly efficient antibiotics onto our skin, which protect us from dangerous bugs. If our skin becomes injured by a small cut, a scratch, or the sting of a mosquito, antibiotic agents secreted in sweat glands, such as dermcidin, rapidly and efficiently kill invaders. In one important aspect, these so-called antimicrobial peptides (AMPs) are even superior to traditional antibiotics: The germs are not capable of developing resistance against them in a rapid fashion. AMPs efficiently prevent this by attacking the Achilles’ heel of the pathogens: The antibiotics pierce their cell membrane, which microbes cannot easily modify. Therefore, AMPs have great potential to form a new generation of antibiotics.
1700 natural AMPs known
“But in order to tailor these therapeutic agents, we first need a detailed understanding of how these natural antibiotics control bugs. Although about 1700 such peptides have been detected so far, we only know very little about their structure and function,” says Bert de Groot, head of the “Computational Biomolecular Dynamics” group at the Max Planck Institute for Biophysical Chemistry. Together with colleagues from Göttingen, Edinburgh (United Kingdom), Tübingen, and Strasbourg he elucidated for the first time what turns dermcidin into such an efficient weapon in the battle against dangerous bugs.
Scientists have known for some time that dermcidin is cleaved in salty, slightly acidic sweat and thus becomes activated. The active peptide then forms tiny channels perforating the cell membrane of microbes, which are stabilized by the zinc ions present in sweat. As a consequence, water and ions uncontrollably flow across the membrane, and the cell's homeostasis and transport processes across the membrane get out of control, eventually leading to the microbes' death.
Dermcidin attacks the invaders' Achilles heel
Through a combination of X-ray crystallography and solid-state nuclear magnetic resonance spectroscopy, scientists around Kornelius Zeth at the Max Planck Institute for Developmental Biology in Tübingen and Burkhard Bechinger from the University of Strasbourg were able to elucidate the atomic structure of this channel. They found that it is unusually long, highly permeable, and adaptable. It thus represents an entirely new class of membrane proteins.
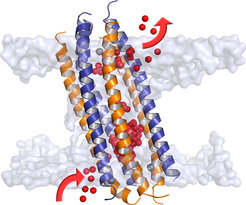
Aided by extensive computer simulations, the team around Bert de Groot watched the channel as it performs its action. Surprisingly, the ions traversed the channel along a very unusual pathway. As chemist Ulrich Zachariae explains: “Our simulations show that the channel sits in the membrane with a tilt. This enables ions to take the side entrances too, and so more ions can cross the pore at the same time.” The simulations could thus explain the high permeation rate of the channel, measured in electrophysiological experiments by Claudia Steinem at the University of Göttingen. Steinem's results also demonstrate that, without zinc ions, the antibiotic fails to perform. Only when zinc and dermcidin act synergistically, channels across the membranes are formed. When the scientists inhibited this interaction through mutation of a certain building block in dermcidin (a histidine amino acid), channel formation was blocked.
Potential for a new class of antibiotics
What is more, dermcidin can adapt to extremely variable types of membranes. “This could explain why active dermcidin is such an efficient broad-spectrum antibiotic fending off bacteria and fungi at the same time. It is active against many well-known pathogens such as Mycobacterium tuberculosis, the causative agent of tuberculosis, or Staphylococcus aureus“, de Groot explains. Multi-resistant strains of Staphylococcus aureus, in particular, have become an increasing threat for hospital patients. They are insensitive towards common antibiotics and, therefore, are highly difficult to treat. According to the Robert Koch Institute in Berlin, merely 2 % of Staphylococcus bacteria showed antibiotic resistance in 1976, while their proportion had risen to 22 % by 2009. Staphylococcus aureus infections can lead to life-threatening diseases like sepsis and pneumonia. The international team of scientists hopes that their results can contribute to the development of a new class of antibiotics that is able to attack such dangerous germs. (uz)






