Transgene parentage modulates amyloid-β load in a mouse model of Alzheimer’s disease
Alzheimer’s disease (AD) is a debilitating and prevalent neurodegenerative disease, particularly in the aging population. One of its major hallmarks is the deposition of amyloid-β (Aβ). To test various clinical interventions and to interrogate the roles of genes in AD progression in preclinical research, animal models of AD have been established, including the most widely used 5xFAD transgenic mouse model.
5xFAD mice rapidly develop amyloid-β (Aβ) plaques due to the expression of a humanized transgene carrying mutated forms of the precursor protein APP and enzyme subunit PSEN1. Age and sex have been known to modulate Aβ plaque load in these mice, however large variabilities sometimes still exist after age- and sex-matching mice in experimental cohorts.
Researchers led by Andrew Sasmita, Constanze Depp, and Klaus-Armin Nave at the Max Planck Institute for Multidisciplinary Sciences in Göttingen, together with colleagues from Munich, Leipzig, and Cambridge (US) have now shown that, in addition to age and sex, the parental source of the 5xFAD transgene determines the severity of Aβ plaque deposition in the offspring.
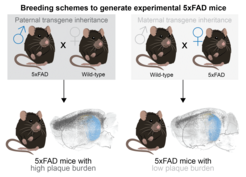
Using quantitative light sheet microscopy, the team discovered that Aβ plaque burden is further modulated by the parental source of a mouse’s transgene: whether the 5xFAD parent is male or female. Mice with a paternal transgene inheritance develop twice the plaque load of mice with a maternal inheritance. This transgene inheritance effect could in fact also be relevant in various transgenic mouse models as the scientists have shown the same effect in another non-AD model using the same promoter, Thy1, which is also used in many other AD models to drive transgene expression.
By controlling breeding schemes of transgenic mice more stringently, variabilities in primary experimental readouts could be better controlled and minimize the need for ample experimental animals. By revealing yet another strong modifier of plaque deposition, the researchers hope to further mitigate large variabilities that would otherwise impede the fast-paced nature of AD research. (aos/cr)


