Cholesterol/Lipids in the Pathology of Neurodegenerative Disorders
Primary defects in lipid metabolism are associated with myelin disease and vice versa, a growing number of neurodegenerative diseases, including multiple sclerosis (MS) and the hereditary leukodystrophy Pelizaeus–Merzbacher disease (PMD), are associated with a perturbed brain lipid metabolism. In the nervous system, the majority of lipids is found in myelin sheaths, a multilayered stack of membranes synthesized by oligodendrocytes. Myelinating oligodendrocytes contribute to axon integrity by providing trophic support and electrical insulation for impulse propagation. Myelination failure is hence associated with axon damage and dysfunction leading to deficits in cognition and motor abilities.
Multiple sclerosis (MS)
Multiple sclerosis (MS) is an inflammatory demyelinating disorder in which remyelination failure contributes to persistent disability.
We investigate the relationship between cholesterol and other lipids, myelination, and neurological parameters in mouse models of demyelination and remyelination. Using mouse models of MS and lipid-based therapies we dissect pathomechanisms and therapeutic strategies (Cholesterol recycling supports myelin repair - press release). However, endogenous repair strategies differ between the acute and chronic phases of myelin disease, and their efficacy attenuates with disease chronicity (Local cholesterol metabolism orchestrates remyelination: Trends in Neurosciences (cell.com)). Moreover, ketone bodies critically influence the CNS energy metabolism, especially during neuroinflammation (Von konservativ bis flexibel | Max-Planck-Institut für Multidisziplinäre Naturwissenschaften (mpg.de)).
In a wide range of neurological disorders including multiple sclerosis (MS) increased vascular permeability has been observed. In MS, the blood-brain barrier (BBB) permeability is increased in both newly forming demyelinating lesions and even in normal appearing white matter BBB disruption could potentially be secondary to pathology.
Pelizaeus–Merzbacher disease (PMD)
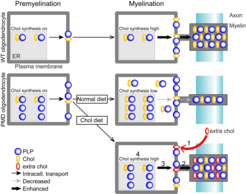
Pelizaeus–Merzbacher disease (PMD) is a leukodystrophy without therapeutic options. In most cases, PMD is caused by a duplication of the X-linked myelin gene PLP1 (proteolipid protein 1). In PMD oligodendrocytes, overexpressed PLP accumulates together with cholesterol which impairs the intracellular transport of proteins and lipids to the growing myelin sheath. In combination with the progressive loss of mutant oligodendrocytes, this leads to dysmyelination and demyelination in PMD. The MRI pattern of diffuse hypomyelination in PMD caused by PLP1 duplication is hence considered consequence of arrested brain maturation and lacks focal or inflammatory demyelination. Despite variable disease severity of PMD duplication patients, hypomyelination improves only to a minor extent over time. At later stages, axon degeneration is also a feature of the PMD pathology, leading to cortical atrophy that likely contributes to neurological impairment.
We hypothesize that the accessibility to the CNS determines the efficacy of lipid treatment. In the CNS, the different lipid classes target distinct aspects of the hypomyelinating pathology, i.e. the primary defect in myelin-forming oligodendrocytes and the subsequent damage of hypo/demyelinated axons. We aim to combine two important therapeutic targets, cholesterol for the support of remyelinating oligodendrocytes and ketone bodies for the metabolic support of the axonal compartment.
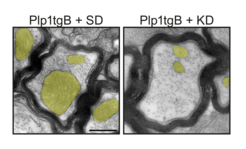
Fig. 1: Ketogenic diet (KD) rescues mitochondria enlargement and ameliorates impulse conduction in a mouse model of PMD (Plp1tgB).