Neuronal structures in color – a super-resolution view into the living brain
Triple STED microscopy enables in vivo super-resolution of synapses by quasi-simultaneous imaging of three different proteins.
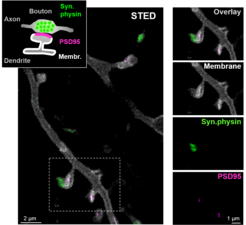
The brain of a mouse consists of almost 100 million nerve cells that are densely packed together with supporting cells. To study these cells, individual cells are specifically labelled with fluorescent dyes. After appropriate excitation with light, such fluorescent dyes emit light themselves that can be detected microscopically. Of particular interest are synapses, the contact sides between two nerve cells where information is transferred from cell to cell and which form the basis of our memory and all neuronal activity. Synapses are only a few tens to a few hundreds of nanometers in size and can be localized using conventional light microscopy. Their exact shape and size, however, can only be imaged in living organisms using super-resolution light microscopy such as STED microscopy. A research group at the Max Planck Institute of Experimental Medicine in Göttingen, led by Katrin Willig, has now developed a method to simultaneously image three different protein structures using STED microscopy in the brain of a living mouse. The study, published in the journal Cell Reports shows that this method can be used, for example, to observe pre- and postsynaptic protein structures with super-resolution in the brain.
With the award of the Nobel Prize in Chemistry 2014 to the Göttingen physicist Stefan Hell for his invention of STED microscopy, super-resolution light microscopy became world-famous and is now widely used in research laboratories worldwide. An advantage of STED microscopy over other super-resolution microscopy techniques such as PALM/STORM is that it can be used to image not only single cells or thin layers, but also structures in dense tissue. Katrin Willig's research group has set out to develop STED microscopy further especially for neurobiological research, and in particular for in vivo microscopy on living organisms. The researchers have now achieved that not only a single fluorescent label can be superresolved, but that three different protein structures, can be imaged in vivo with super-resolution STED microscopy. Two of the labels are spectrally separated as in conventional fluorescence microscopes, i.e. a green light-emitting fluorescent dye is combined with one that emits yellow light. For the third label, the researchers use a fluorescent dye that emits in the same yellow/green spectral range but can be switched on and off reversibly with light as needed. The switching process is accelerated by parallelization so that all three proteins can be displayed quasi-simultaneously. This is particularly important in living organisms, where small changes occur continuously due to spontaneous or induced processes. In the now published work, the researchers labeled synaptic vesicles that are abundant in the presynapse in so-called synaptic boutons (Figure 1). In addition, the membrane of the postsynaptic neuron and the postsynaptic membrane thickening were highlighted. This principle can now be easily transferred to other synaptic proteins or to proteins of supporting cells. In the future, this technique will be used to study how synapses change in shape and structure over time, for example after learning processes.