No energy flux through the chemical bond!
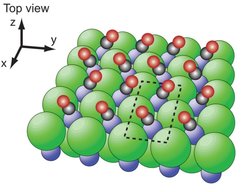
The exchange of vibrational energy between molecules is usually a consequence of the anharmonic interatomic forces coupling the motion of one molecule to the other. For molecules bound to a surface, for example, the anharmonic interactions with the substrate are so efficient that the adsorbate’s vibrational motion is damped within a few nanoseconds. Researchers of the MPI for Biophysical Chemistry in Göttingen, in collaboration with Leiden University (Netherlands) and colleagues at the National Institute of Standards and Technology, Boulder, Colorado (United States) have now discovered that for oscillating CO molecules adsorbed in a monolayer on a sodium chloride (NaCl) crystal surface this energy loss mechanism is completely unimportant.
The CO molecules are on the one hand sufficiently strongly bound to the solid to form samples which are stable over long periods of time. On the other hand, the contribution of this bond to draining vibrational energy from the CO to the NaCl lattice vibrations is negligible. In fact, the interactions between neighboring CO molecules are stronger than CO-NaCl interactions, leading to conditions were the vibrational energy hops from molecule to molecule and in this way freely migrates within the monolayer for tens of milliseconds. The vibrational lifetime is limited by a loss mechanism that is similar to a radio transmitter experiencing damping due to electromagnetic interaction with the Earth. The corresponding theory has its origin in Arnold Sommerfeld’s work from 1909. In the case of CO/NaCl, the oscillating CO molecules act like dipole antennas being damped by the electromagnetic interactions with the NaCl crystal.
