Probing surface chemistry with ion imaging
Group Leaders: Theofanis Kitsopoulos and Alec M. Wodtke
Group Members: Dmitriy Borodin, Jan Fingerhut, Michael Schwarzer
Collaborator: Barratt Park
Our group implements slice imaging to measure catalytic rates for site-specific elementary reactions thus offering remarkable opportunities to advance our fundamental understanding of heterogeneous catalysis. Knowledge of elementary chemical reaction mechanisms in heterogeneous catalysis underlies our ability to construct comprehensive kinetic models for many such important chemical processes, in order to optimise them.
Our proposed strategy makes the formidable task of describing site-specific chemical reaction mechanisms and elementary rates in heterogeneous catalysis facile, while its necessity we justified Nature 2018,[1] on the prototypical CO oxidation reaction on Pt by demonstrating that 40 years of traditional experimentation led to false interpretation of the reaction mechanism.
Our aim is to characterize the important factors that influence the kinetics of elementary reactions at surfaces, e.g. the chemical nature of the catalyst and the geometry of the active site (stereodynamics). We chose elementary reactions involving C, H, O, N, as these are important in many key industries, such as the methane reforming, syngas, fuel cells, Fischer-Tropsch synthesis and the Haber-Bosch process. Our strategy is that of a “bottom-up” approach to catalysis, i.e., building and understanding complex heterogeneous chemical catalysis, from the site-specific kinetics of the elementary building block reactions. Our measurements, serve for benchmarking first principles calculations of reaction rates in surface chemistry. Our methodology measures the kinetics in the ms regime with temperatures in the 200 to 1000 K range, i.e, conditions more relevant to industrial conditions.
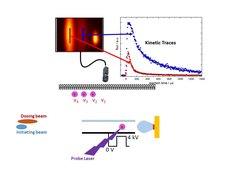
Two temporally short-pulsed molecular beams (duration ~ 20 μs) are incident on the surface at 0° (dosing pulse) and 30° (probe pulse) from the surface normal. Variation of the relative repetition-rates of the pulsed nozzles controls the concentrations of the reactants on the surface. Product molecules are non-resonantly ionized using 50 fs light pulses from a Ti:Sapphire laser operating at 800 nm. The ions generated are extracted using a pulsed homogeneous electric field, mass resolved using an ion-gate, and detected using a position sensitive imaging detector. Ions from product molecules with different velocities appear at different positions in the image.[2, 3] Velocity space images are recorded as a function of the kinetic timing, i.e. the delay time between the probe pulse which initiates the reaction and the Ti:Sapphire laser pulse, which detects the products. We analyse this data to obtain the kinetic trace. We integrate the signal intensity (proportional to product density) within narrow velocity windows and plot this as a function of the kinetic timing. By knowing the distance from the surface to the laser focus and the velocity, we then calculate and subtract the time needed for the product molecules to reach the laser pulse from the surface. We also use the measured velocity to convert the product density to flux. This is repeated analysing velocity images at all measured values of the kinetic timing leading us to results like those shown in the inset: kinetic traces for two reaction channels producing products with very different velocities.
1. Neugebohren, J., et al., Velocity-resolved kinetics of site-specific carbon monoxide oxidation on platinum surfaces. Nature, 2018. 558(7709): p. 280-283.
2. Harding, D.J., et al., Using Ion Imaging to Measure Velocity Distributions in Surface Scattering Experiments. Journal of Physical Chemistry A, 2015. 119(50): p. 12255-12262.
3. Harding, D.J., et al., Ion and velocity map imaging for surface dynamics and kinetics. Journal of Chemical Physics, 2017. 147(1): p. 7.

