Overview
Human life starts with the fertilization of an egg cell by sperm. During fertilization, the genetic material of the mother and the father – which is stored in the form of chromosomes – is united. Egg and sperm differ from all other cells in our body in one central aspect: They contain only half the number of chromosomes. Normally, each chromosome is present in two copies. In contrast, the egg has only one of the two copies of each of the mother’s chromosomes, and the sperm contains only one of the two copies of each of the father’s chromosomes. An egg develops out of a progenitor cell, the oocyte, which still possesses two copies of each chromosome. To become a fertilizable egg, the oocyte has to eliminate one of the two copies. This happens once every menstrual cycle through a specialized cell division called meiosis. During meiosis, one of the two copies of each chromosome is eliminated from the oocyte and discarded in a small cell called polar body. Often, this does not work reliably, resulting in an egg with the wrong number of chromosomes. If such an egg is fertilized, the embryo will also have the wrong number of chromosomes. In most cases, an embryo with the wrong number of chromosomes will die. In other cases, the embryo may be viable, but it will suffer from congenital disorders such as Down syndrome, which is caused by an extra copy of chromosome 21. Eggs with the wrong number of chromosomes become more frequent as women get older. This is called the maternal age effect. Despite decades of work, we still know relatively little about meiosis in mammalian oocytes. Especially human oocytes have hardly been studied. At the same time, fertility problems become more and more prominent in our society, also because childbearing is more frequently postponed until later in life. To improve fertility treatments it is essential that we learn more about the mechanisms that govern accurate progression through meiosis and that we analyze the causes of chromosome segregation errors in mammalian oocytes. Thus, research into mammalian oocytes, with its many open questions and medical implications, has enormous potential to grow and will remain an attractive field for a long time to come.
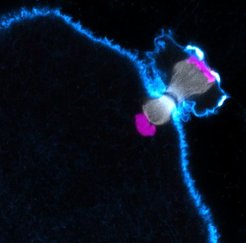
Chromosome segregation is error-prone
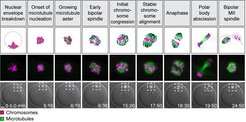
To find out why eggs frequently have the wrong number of chromosomes, we need to investigate how eggs develop. Our aim is to understand how the chromosomes become prepared for elimination into the polar body, and how the machinery distributing the chromosomes between polar body and egg works. This machinery is called the microtubule spindle and consists of protein fibers, which separate the chromosomes. If the spindle is abnormal, chromosomes cannot be separated accurately. Indeed, our work has revealed that chromosomes are frequently abnormally attached to the spindle in human oocytes, which may contribute to the high error rates in meiosis. We are also investigating the causes of declining female fertility as women get older. We have found that chromosomes in human oocytes are disintegrating as women age. This leads to more and more errors during the segregation of chromosomes as women get older. This decline in oocyte quality may be due to the fact that eggs are as old as the woman: A 40-year-old woman has 40-year-old eggs.
New tools to study meiosis
To have a solid foundation for future research, we are developing new tools to study meiosis in mammalian oocytes. For instance, we have been able to carry out the first so-called high content screen for genes controlling meiosis in mammals. Moreover, we developed a new method for the acute degradation of endogenous proteins, called Trim-Away. We have also been able to establish methods that now allow us, for the first time, to study the causes of chromosome distribution errors directly in live human oocytes. This opened an exciting new area of research in our lab that we plan to expand significantly in the future.



