How molecular motors start the spliceosome
The spliceosome is the molecular machine in our cells that puts the blueprints for proteins into a readable form. Researchers at the Max Planck Institute (MPI) for Multidisciplinary Sciences in Göttingen (Germany) and the Institute for Cancer Research (ICR) in London (UK) have now uncovered the crucial step that switches on the spliceosome. As they have shown, this macromolecular machine is activated by two molecular motors. The findings from the study could provide new approaches to improve potential cancer drugs that target the splicing process.
For a cell to produce proteins, it must first convert the blueprints encoded in our genes into a readable form. To do this, the gene is transcribed into a precursor of the messenger RNA (mRNA). This pre-mRNA, however, does not yet contain the blueprints in one piece. In a complicated process, the spliceosome has to cut out sections that are not needed and reassemble the information-bearing sections – the exons. This process is called splicing. Splicing has a decisive advantage: The exons can be assembled in different ways according to need. This way, in humans, about 20,000 genes provide the building instructions for more than 100,000 different proteins.
The most complex machine in our cells
“The spliceosome stands as one of the most intricate and dynamic molecular machines from our cells. With over 150 proteins belonging to the various spliceosome states, its complexity seems unparalleled,” explains Vladimir Pena, who led the research, first as a group leader at the MPI for Multidisciplinary Sciences and then as a professor at the ICR. “During splicing, the spliceosome undergoes numerous steps that change its structure and composition. These steps are driven by molecular motors called helicases. The complex structure of the spliceosome presents significant obstacles in the study and comprehension of how its constituent parts come together and operate as a cohesive unit within the splicing mechanism.”
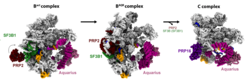
Into the heart of the spliceosome
Using cryo-electron microscopy and biochemical methods, Pena’s team succeeded to “capture” the spliceosome at near atomic resolution in the middle of the switch-on process. In the center of this process is a component of the spliceosome called SF3B1, which is essential for the machinery’s activation.
“We discovered that the spliceosome in humans can only be started with the help of two helicases, PRP2 and Aquarius, altering SF3B1,” Pena said. “Helicases are a special type of protein. They convert chemical energy stored in ATP – a chemical molecule that provides energy in living cells – into mechanical work. In other words, they are autonomous motors driven by the ATP battery.”
The researchers further showed that PRP2 interacts with SF3B1 in a way never before observed for helicases. Constantin Cretu, one of the first authors of the study, describes this distinctive feature: “Instead of binding to the outside of the spliceosome like other helicases, PRP2 migrates along the RNA strand into its ‘heart’. In the process, PRP2 rearranges the spliceosome’s structure and composition and puts the molecular machine into an active state.” The details in the operation of a splicing helicase were revealed with unprecedented clarity, and suggest that several other helicases might act on the spliceosome in similar ways. However, in contrast to PRP2, Aquarius belongs to a distinct class and carries out its functions from the surface of the spliceosome.
PRP2 at work
“With our experiments, we have visualized a crucial new intermediate step in the cycle of the spliceosome that has not been described before,” says Jana Schmitzová, also first author of the publication. “As a result, we have uncovered important rearrangements of proteins: Some are sorted out and replaced, others change their position. It is fascinating to see how all the protein players interact in such an orchestrated way.”
Splicing errors trigger cancer
In some types of cancer, like leukemia, uveal melanoma, pancreatic cancer, and prostate cancer, SF3B1 is the component of the spliceosome that most often exhibits mutations. This spliceosome component is a well-known target for antitumor compounds, some of them investigated by Pena’s team. “We hope our findings will stimulate new research to elucidate the causes of cancer development further. Thus, it could help develop more efficient anticancer drugs that target the splicing process,” Pena remarks. (kr/cr)












