Chromatin dynamics
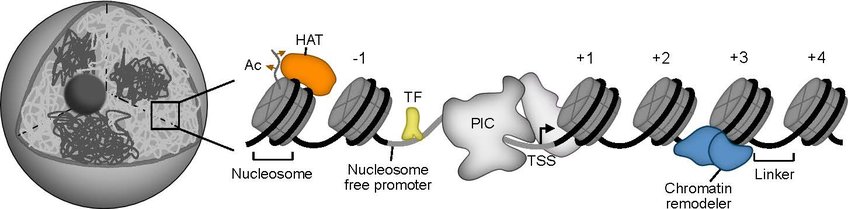
Gene expression is one of the most fundamental processes in our cells, where DNA is transcribed into RNA and then translated into protein. Dysregulation in gene expression often leads to severe diseases such as cancer. In the first step of gene expression, our genetic material must be made accessible. However, our genome is packaged and protected by nucleosomes. Nucleosomes consist of 147 base pairs of DNA wrapped around eight histone proteins and are connected by short DNA segments of 20 to 30 base pairs. The entirety of the nucleosomes forms the chromatin in our cell nucleus.
Nucleosomes cover 75 to 80 percent of our genome and thus frequently block access to promoter sequences, which must be free of nucleosomes to be bound by the transcription machinery. Therefore, a group of SNF2-type helicases positions or removes nucleosomes using ATP. These so-called ATP-dependent chromatin remodelers are often large multi-protein complexes that interact with transcription factors or epigenetic modifications to become locally active. However, the molecular details of these interactions are often unclear.
The aim of our research is to decipher the molecular functions and interaction networks of chromatin remodelers. We use a complex reconstitution approach in which chromatin is recreated genome-wide with purified proteins. This allows us to study the function of chromatin remodelers and their interaction partners in a complex but controlled chromatin environment. As an analytical method, we use Next Generation Sequencing techniques to map the exact nucleosome positions or specific, epigenetic remodeler-nucleosome interactions. With this innovative approach, we hope to better understand the role of chromatin remodelers in gene regulation and cancer development.
