Synaptische Metallionen-Dynamiken und Signalwirkung
Eine deutsche Beschreibung unserer Forschung wird in Kürze verfügbar sein.
Transition metal ions (metals) such as copper, iron, and zinc play key roles in many cellular processes. In addition to the clearly established roles as co-factors of enzymes and various other proteins, metals are emerging as major effector of molecular signaling. Our broad research interest is to understand the role of metal ions in neuronal cell biology and signaling. As metal homeostasis is impaired in neurodegenerative and cognitive disorders, our fundamental investigations on metal dynamics and regulation aim to get insights on the aetiology of disease mechanisms.
In general, metals exhibit high binding affinity towards proteins and small molecules, and are considered to be mostly in the ligand-bound state, in contrast to the negligible proportion of free metal ions inside the cell. However, rapid changes in the membrane potential and pH by synaptic activity produce transient changes in the free metal ion concentration in neuronal compartments, which may trigger fast signaling events mediated by metal ions in the neuron. Despite the abundance of metal binding proteins, metal dynamics and signalling is largely unexplored owing to technological limitations. Using recently developed imaging tools including those from our group, we study the interaction between neuronal activity and cell biological processes mediated by metal ions, which is critical to understand why metal homeostasis is disturbed in many neuronal disorders.

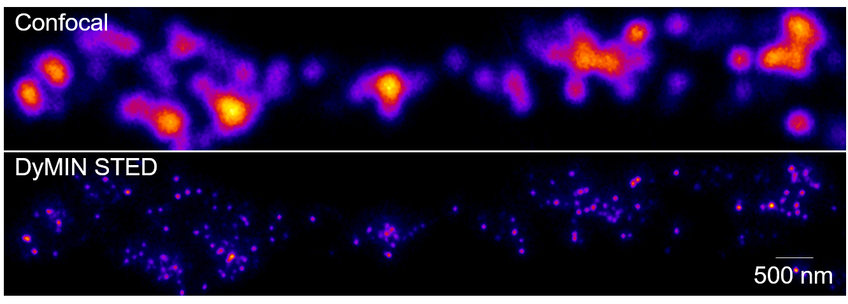
Our recent work (Upmanyu et al, 2022, Neuron) revealed a novel role for zinc in synaptic transmission. Using recently developed super resolution microscopy methods such as DyMIN STED and MINSTED, we found that zinc transporter 3 (ZnT3), which is responsible for loading zinc into synaptic vesicles, is one of the most abundant vesicular neurotransmitter transporters in the brain and co-localized on the same vesicles that store glutamate. With the help of novel biochemical assays and electrophysiology, we showed that cytoplasmic zinc regulates vesicular glutamate content and the synaptic quantal size through ZnT3, demonstrating a direct role for zinc in the determination of synaptic strength. Currently, we try to understand the holistic molecular scheme of zinc biology at the synaptic terminal.
In order to build a comprehensive understanding of neuronal metal homeostasis, we employ Minflux super resolution nanoscopy, which delivers 3D multicolor nanometer resolution in cells, to determine the nanoscale organization of endogenous metal transporters on the cell membrane and subcellular organelles in the intact nervous system. By combining the above approach with DNA-PAINT acquisition, we enhance the multiplexing capabilities to achieve realistic in situ structural biology of metal transporters, which is critical to understand and interrogate the local metal dynamics in neurons.
Legend of the image above: A straightened neuronal dendrite showing super resolved axon terminals containing individual synaptic vesicles labelled against zinc transporter 3, a protein responsible for zinc uptake into the vesicles.

