Cholesterol is Rate-Limiting for Myelination
To analyze the function of cholesterol in myelin formation, we analyzed squalene synthase conditional mutant mice that lack cholesterol synthesis specifically in myelin-forming glial cells. At the age of about two weeks, coinciding with the normal peak of CNS myelination, mutant mice develop motor function deficits. Mutants showed ataxia, initiation tremor, and impaired control of hindlimb movements. About one third of mutant mice even died between 20 and 30 days of age. Remarkably, we rarely observed the death of mice that survived past the first month, suggesting that older animals have overcome a critical period in the course of this disorder.

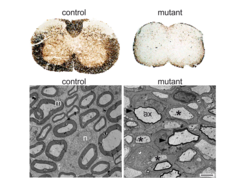
Histochemical analysis revealed that conditional mutants are severely dysmyelinated in the CNS and PNS (Fig. 2). By ultrastructural analysis we found that hypomyelination is most severe in spinal cord. In white matter fiber tracts of spinal cord of mutant mice, most axons displayed abnormally thin myelin sheaths or were unmyelinated. Surprisingly, mutant oligodendrocyte cell bodies showed a normal morphology and appeared healthy.
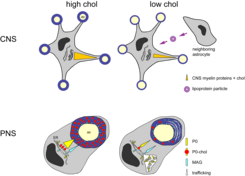
Genetic disruption of the cholesterol biosynthesis pathway in oligodendrocytes was expected to alter the lipid composition of oligodendrocytes and consequently of myelin itself. However, the lipid profile of purified CNS myelin was unchanged (by Nano-ESI-MS/MSmass spectrometry). Surprisingly, cholesterol was present in myelin derived from mutant mice. Importantly, a direct comparison of cholesterol levels in total brain versus purified myelin revealed a major "enrichment" of cholesterol in myelin, both in mutant mice and in controls. This strongly suggests that cholesterol incorporation and enrichment is a requirement for myelin membrane growth, independent of the cholesterol source.
In contrast in the PNS, the myelin composition changed when SQS was inactivated in Schwann cells. Increased levels of proteins located in uncompact myelin matched the occurrence of stretches of uncompacted myelin (Fig. 3). The cholesterol content in myelin was reduced to about 60% of control levels. We found that the major protein of the peripheral myelin P0 associates with cholesterol. The ER exit of P0 and its trafficking to the plasma membrane depends on cholesterol.
Taken together, cholesterol is an essential component of myelin, as there is no assembly of compact myelin at low cholesterol levels. Therefore, cholesterol incorporation emerges as an essential and rate-limiting factor for myelin membrane growth in the central peripheral nervous system.