Playing squash on the atomic scale
Fig.1: Playing squash with molecules.
Although not Olympic, squash is a popular sport where two players strike a little ball against the walls of the squash court. The main idea of the game is easy to understand: The opponents try to anticipate the location where the ball will be by estimating incidence angle, velocity and spin of the ball struck by the other player towards the wall.
The same happens countless times on the atomic scale in our everyday life, invisible to our eyes. Molecules in the air collide with surfaces of different walls. However, the situation is considerably more complicated than in the macroscopic picture. Besides direct backscattering, molecules can also simply stick to the surface: a process which is called adsorption. Once the molecule sticks to the surface, it might be activated for chemical reactions. This is a fundamental step in heterogeneous catalysis, which describes reactions of gas phase molecules at surfaces and is of outstanding importance for the production of chemical compounds on an industrial scale. In analogy to the picture drawn above, one can imagine that the behavior of molecules at surfaces depends crucially on the characteristics of both the molecule (ball) and the surface (wall). The shape of the molecule, which can be imagined consisting of atoms connected by a chemical bond, determines among others essentially the molecule-surface interaction.
The challenge of modern science is to make these microscopic processes visible. Using high resolution laser systems, the vibration, rotation, translation, and orientation of molecules can be both probed and prepared. Thus, the exchange of energy, photons, and particles as well as chemical reactions on surfaces – all elementary processes that strongly depend on the molecule’s orientation – can be studied precisely in the lab. Among all these phenomena, electron transfer reactions are of particular interest because of their importance in a remarkably wide range of phenomena.
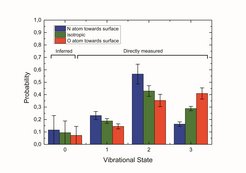
In our recent paper highlighted here, we have examined electron transfer reactions at surfaces, which control the change of oxidation state in surface chemistry, a critical factor explaining catalytic activity and selectivity. We report remarkably clear observations of a strong orientation dependence of the electron transfer reaction probability on the vibrational relaxation probability of a diatomic molecule interacting with a noble metal surface (see Fig. 2).

